Você já comprou um item na loja e, ao chegar em casa, percebeu que a cor não combinava com a decoração? A verdade é que a cor não mudou; foi a maneira como a luz influenciou sua percepção. Para controlar a cor, é crucial entender as diferenças sutis entre elas e o impacto da iluminação na aparência dos objetos. Na segunda parte desta série, exploraremos como a luz afeta a cor que percebemos e a importância de uma iluminação controlada em um programa de tolerância bem-sucedido.
Cor é Luz, e Luz é Energia
A luz não é uma entidade única; existem diversos tipos de luz, e cada um distribui sua energia de maneira distinta. A nossa percepção de cor é intensamente influenciada pela fonte de luz que incide sobre um objeto. Para estabelecer um programa eficaz de tolerância de cores, é fundamental compreender o papel da luz na percepção da cor.

Para estabelecer um bom programa de tolerância, você precisa entender o papel da luz na cor.
Em 1670, Sir Isaac Newton realizou um experimento que colocou o fenômeno da cor em palavras. Ele pendurou um prisma em uma sala escura e introduziu a luz solar através de uma pequena fenda. À medida que a luz passava pelo pedaço triangular de vidro, ele observou que ele refratado em uma série de cores sobre a parede: um arco-íris. O prisma dobrou os componentes individuais da luz branca para que cada um pudesse se tornar visível.
A partir deste experimento, Newton teorizou que a luz branca é realmente composta de muitos tipos diferentes de luz: vermelho, laranja, amarelo, verde, azul, ciano, violeta. Ele estava certo.
A luz produz energia eletromagnética com muitos comprimentos de onda diferentes. Na extremidade curta - 400 nanômetros - a luz é violeta. À medida que o comprimento de onda se torna mais longo, até 700 nanômetros, a luz passa do violeta para o azul, para o verde... amarelo para laranja e vermelho. Newton provou que a luz branca não é branca. Na verdade, é composta por todos os diferentes tipos de luz... Energia eletromagnética em intervalos entre 400 e 700 nanômetros.
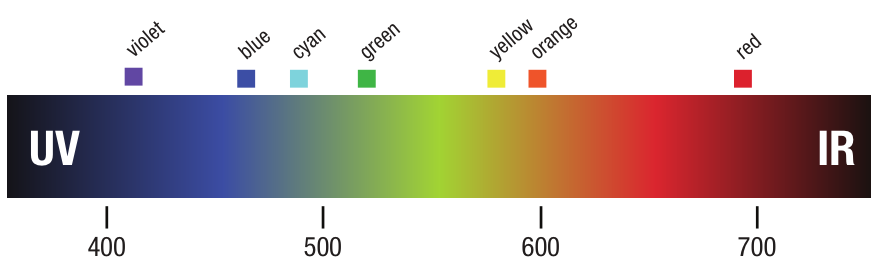
Diferentes Fontes de Luz
Desde a época de Newton, as fontes de luz evoluíram significativamente. Atualmente, dispomos de diversas opções, como luz incandescente, fluorescente e LED, cada uma emitindo energia em diferentes pontos do espectro visível. Cada tipo de luz pode impactar a percepção das cores de maneira única.
Illuminante D65 (Luz do Dia)

Iluminante D65 (Luz do Dia) representa a luz do dia em temperatura de cor de 6500K, onde a energia é mais intensa na parte azul do espectro. Como você pode ver, há muita pouca energia no lado esquerdo do espectro; na região violeta em torno de 400nm. A energia do dia atinge o pico na porção azul, depois diminui à medida que o comprimento de onda fica mais longo. A 700 nanômetros há pouca energia vermelha. Mas, há muitos tipos de luz do dia. D65 descreve a luz do dia do meio-dia, onde o sol está escondido atrás de um edifício, e tudo parece mais azul porque o dossel azul do céu está fornecendo iluminação. A luz do dia D75 é ainda mais azul na sombra. De manhã e à noite, o sol está no horizonte. A esta hora do dia, a luz do dia é bastante vermelha.
Illuminant A (Incandescente) 2856 K

Iluminante A (Incandescente 2856K) representa a luz incandescente, que tende a realçar tons vermelhos e amarelos, alterando a percepção das cores. Uma lâmpada de tungstênio queima em cerca de 2800 Kelvin. Tem muito pouca energia nos comprimentos de onda violeta ou azul, mas a energia continua a aumentar em quase uma inclinação constante para 700 nanômetros, o que é uma abundância de vermelho. Enquanto objetos iluminados pela luz do dia parecem azulados, eles se deslocam para vermelho sob iluminação incandescente.
Illuminant F2 (Fluorescente Branco Frio) 4100 K

Iluminante F2 (Fluorescente Branco Frio 4100K) esta luz apresenta uma predominância de amarelo-esverdeado, o que pode distorcer a percepção das cores. Uma lâmpada fluorescente que tem uma temperatura de cor de 4100 Kelvin. A fluorescente branca fria cai entre a luz do dia azul e a incandescente vermelha com predominância em amarelo esverdeado e fraqueza em violeta, azul e vermelho. Os picos são emissões de vapor de mercúrio, parte do projeto da lâmpada. Esses picos causam estragos ao julgar a cor sob uma fonte de luz fluorescente.
Fontes de luz comuns
Aqui está uma visualização de quanto a temperatura da luz afeta o que vemos. Esta cabine de visualização está iluminando a mesma cena com diferentes fontes de luz.

Na primeira imagem, a luz incandescente aumenta o vermelho. A imagem do meio é tirada sob iluminação fluorescente, fraca em azul e vermelho com predominância em verde. A luz do dia na última imagem produz energia azul, o que faz com que os objetos assumam uma tonalidade azulada.
À medida que um objeto interage com a luz, ele só pode refletir a luz que existe. Objetos não criam luz; eles refletem a luz que vem da fonte. Assim como a fonte muda, assim como a reflexão (e cor) que vemos a partir do objeto.
Aprendizados importantes para tolerância
- Ao comparar cores, você deve estar ciente (e no controle de) sua fonte de luz.
- Normalmente, a maioria das indústrias especifica a luz padrão sob a qual os materiais devem ser visualizados. Certifique-se de perguntar qual fonte de luz usar ou usar a iluminação padrão para sua indústria se você não tiver certeza.
- Para medir e avaliar a cor sob a mesma iluminação, você deve selecionar o mesmo illuminant em seu espectrofotômetro, software de tolerância e cabine de luz para consistência.
- Comunique-se com seus fornecedores e clientes para garantir que eles estejam seguindo os mesmos procedimentos de iluminação.
Para Ler Mais...
Na terceira e última parte desta série, definimos a diferença entre um espaço de cor e um modelo de cor, e introduzimos os métodos de tolerância mais usados - Tolerância Parte 3: Espaço de cor vs. Tolerância de Cores.
Saiba mais sobre A Ciência Por Trás da Avaliação Visual.
